INDEPENDENT DEALER
Saint George, UT | (208) 589-4660
Conventional Vs. Synthetics
AMSOIL synthetic lubricants deliver wear protection, engine cleanliness and fuel efficiency conventional oils simply can’t match. They help your vehicles run better and last longer.
Oil, whether synthetic or petroleum-based, consists of molecular chains of hydrogen and carbon atoms, referred to as hydrocarbons. Petroleum crude oil is a thick, highly flammable dark-brown or greenish liquid with high energy densities. Many contaminating elements exist in this complex mixture of hydrocarbons, including sulfur, nitrogen, oxygen and metal components such as nickel or vanadium. Petroleum crude oil is the raw materialused for a wide variety of petrochemicals, including solvents, fertilizers, plastics and lubricants.
The oil refining process separates the various types of molecules in the oil by weight, resulting in a concentrated batch suitable for today’s uses such as gasoline, LPG, kerosene or base oils for lubricants. The chemical composition of conventional motor oil can vary substantially and depends on the raw crude oil refining process.
While petroleum base oils are refined, synthetic base oils are manufactured and can achieve a higher performance level. Synthetic oil is chemically engineered for a certain molecular composition with a tailored and uniform structure. Such fine-tuned control over the final molecular composition of synthetic oils is the key to the superior performance properties of these fluids. Designing molecular structures in a planned and orderly fashion results in molecules, and an end-product, that are far more stable than their refined petroleum counterparts.
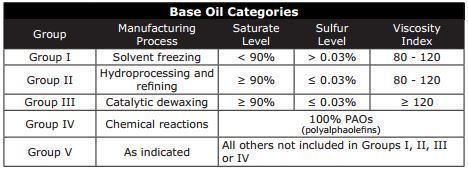
Base Oil Groups
The entire range of base oils, including conventional petroleum products, are divided into five groups based on the level of saturates (saturated molecules), sulfur and viscosity index. In general, the chemical composition and performance properties of the base oil categories improve with advancing group number. For instance, Group I has a lower concentration of saturates than Group II, while Group II has a lower concentration of saturatesthan Group III base oils. Today, Group III, Group IV and Group V base oils are considered synthetic.
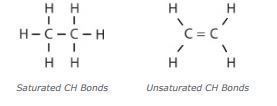
Saturated molecules contain a higher percentage of carbon-hydrogen (CH) bonds, which limits the available sites to which other, harmful molecules can attach. When other molecules, like oxygen, attach to oil molecules, they break down the molecular composition of the oil and weaken its performance. Saturated molecules are beneficial in lubricating fluids because they remain stable longer, resulting in a more durable lubricant. Unsaturated molecules have fewer single carbon-hydrogen bonds and are therefore less stable.
Sulfur is a naturally occurring, inorganic element that readily reacts with oxygen molecules and is detrimental to oil performance. Synthetic base oils have less sulfur than conventional base oils.
Viscosity index refers to the temperature-viscosity relationship of lubricating fluids. Oils with a high viscosity index (VI) are less affected by temperature; those with low VI are affected more. Oils with a VI less than 120 (Groups I & II) are more susceptible to viscosity variance due to temperature. The viscosity index of synthetic base oils is higher than that of conventional petroleum base oils.
Pure, Uniform Molecules Form Strong, Stable Lubricants
Petroleum oils have molecular structures that are randomly organized and, consequently, have limited performance abilities. Their varied and inconsistent molecular structure results in less film strength and lubricity. Their paraffinic wax content also makes them more susceptible to viscosity variance and cold-temperature flow problems.
On the other hand, synthetic base oil molecules are chemically controlled, which provides increased film strength and lubricity over petroleum oils.
The performance qualities of base oils have a marked impact on the performance qualities of the finished product. Synthetic base oils provide key features and customer benefits including better wear protection, more horsepower, increased engine cleanliness, improved fuel economy, easier cold starts and longer oil life.
COLD
36% EASIER AND FASTER COLD STARTS
When the temperature drops, motor oils thicken and move slower, causing parts of your engine to remain unprotected for a short period of time. AMSOIL is more fluid at cold temperatures than the tested conventional oil, giving you 36% easier and faster cold starts*. This means that it can reach vital components faster, providing more immediate engine protection and reduced wear.
*Supported by Cold Crank Simulator test (ASTM D5293)
HOT
5 TIMES CLEANER ENGINES IN HOTTER CONDITIONS
In order to keep on pace with the increasingly strict fuel economy standards, vehicle manufacturers are designing engines differently, causing them to run hotter than ever before. The average operating temperature is up to 235°F, and even higher under heavy loads. This heat can quickly break down the oil and cause oxidation, sludge and deposits.
Recent tests show AMSOIL keeps engines 81% cleaner* (5 times cleaner) than conventional oil by better resisting the formation of deposits.
*Supported by TEOST 33C test (ASTM D6335)
ENVIRONMENTAL CONSERVATION
AMSOIL synthetic motor oils are designed to reduce emissions, last longer and reduce fuel consumption – all features that help prevent pollution. They resist volatilization (burn-off) better than conventional oils, which cuts tailpipe emissions, while extended-drain AMSOIL synthetic oils generate less waste oil and packaging waste since motorists use less oil throughout the year.
FUEL
MAXIMIZE FUEL ECONOMY
The base oils used in conventional oils contain molecules of different sizes and weights as well as unwanted materials, like wax. This typically requires more energy to circulate throughout your engine, which can reduce fuel economy. Conversely, AMSOIL synthetic motor oils are formulated using molecularly uniform synthetic base oils that slip easily over one another and are free of unwanted materials. They help reduce energy lost to friction and maximize fuel economy.
OIL
38% LESS OIL CONSUMPTION
If enough motor oil is consumed, eventually there may not be enough to reach all the complex parts of your engine, which can potentially cause damage.
AMSOIL Signature Series 5W-30 showed 38% less oil consumption* than the tested conventional oil, requiring less-frequent top-offs.
*Supported by NOACK Volatility test (ASTM D5800)



